NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements: NCERT Solutions for Class 11 Chemistry Chapter 11 cover questions for those topics that are vital for the exams. They help students to understand complex concepts in the subject comprehensively. So, teachers must recommend to students to study from NCERT solutions, particularly for a subject like Chemistry, so that they can acquaint themselves with the topics.
Moreover, NCERT Solutions for Class 11 Chemistry Chapter 11 also give questions at the end of each chapter. And by answering them, you will get enough practice to face your exams.
NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
NCERT Solutions for Class 11 Chemistry Chapter 11 is as per the syllabus. The creators of the books have arranged each chapter as it appears in the textbooks. They are available as PDF, and you can download a copy from the websites that host them.
NCERT Solutions for Class 11 Chemistry Chapter 11 has two parts. Each part explains a concept related to the atoms, molecules, elements, and the periodic table. Additionally, it also tells you about the different states of elements and compounds. You can even other concepts like equilibrium and thermodynamics of elements.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements from below.
NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2020/09/chapter_11_the_p-block_elements.pdf”, “#example1”);
What will you learn in NCERT Class 11 Chemistry Chapter 11 The p-Block Elements?
The current chapter talks about p-block elements. P-block elements range from Group 13 to Group 18 in the periodic table and belong to the Boron family. The chapter emphasizes the atomic structure of the elements and their physical properties. It also talks about electronegativity and how it decreases from Boron to Aluminum and increases from thereon.
Group 13 – Boron
Boron is a strong element belonging to Group 13. It has a high melting temperature, owing to its crystalline lattice structure. However, it is not a metal. Others from the Boron family are soft metals with low melting points and high electrical conductivity.
When Boron mixes with other elements such as hydrochloric acid and water, you get different compounds like Borax and Orthoboric acid. While Borax has a white crystalline-like structure, orthoboric acid is a liquified solution of Borax. Borax is soft and soapy and finds uses in making borosilicate glazes that can resist heat. The compound also has applications in pottery for making enamels and glazes.
Similarly, when the element mixes with hydrogen compounds, it forms diborane. Diborane is a colorless gas with high toxicity.
It catches fire instantly when you expose it to the surrounding air. So, it is highly flammable.
Group 14 – Carbon
Group 14 in the periodic table consists of elements like Carbon, Silicon, Germanium, Tin, and Lead.
Carbon is an abundant element that exists in different forms, including coal, diamond, and graphite.
All the elements that form Group 14 are solids, and carbon is no exception to this rule. It has a small atomic size and high electronegativity.
Carbon exists in two forms, namely, amorphous and crystalline. Diamond and graphite are crystalline forms of carbon and find various uses, such as for the sharpening of tools used in the manufacture of dyes. It also finds applications in making tungsten filaments.
Graphite is a soft and slippery element that finds uses in lubricating machines that run at high temperatures.
Other forms of carbon include wood charcoal and animal charcoal.
Carbon has hundreds of other uses. For instance, activated charcoal can absorb poisonous gases. So, it finds applications in gas masks. It also finds uses in the decolorization of sugar.
Likewise, using carbon, you can produce black ink. Carbon also finds applications in the manufacture of automobile tyres.
Coke, a form of carbon, finds uses in metallurgical processes as a reducing agent.
Carbon can form hundreds of different compounds when mixed with other substances.
For instance, when mixed with Oxygen, Carbon forms two prominent compounds that you can find in everyday life, namely, carbon monoxide and carbon dioxide. Both these compounds differ drastically in terms of properties. While carbon monoxide is highly poisonous, carbon dioxide is less toxic. Similarly, carbon monoxide is highly porous and insoluble in water, while carbon dioxide is slightly soluble in water and forms carbonic acid when it dissolves.
Benefits of NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
There are hundreds of benefits of studying NCERT Solutions for Class 11 Chemistry Chapter 11, which is why those who aspire to come out on top prefer them. Listed below are a few benefits of preparing for NCERT Solutions for Class 11 Chemistry Chapter 11.
1. Prepared by Experts
Teachers with extensive knowledge prepare NCERT solutions. They have only one goal in mind – To provide students of class 11 with quality educational content. So, NCERT solutions for Chemistry are concise as well as detailed and offer students with enough information to crack the exams.
2. NCERT Solutions Enable Students to Test their Knowledge
NCERT Solutions for Class 11 Chemistry Chapter 11 come with questions at the end of each chapter. So, it allows students to evaluate their knowledge of the different concepts that appear in the chapter.
3. NCERT Solutions Function as Revision Materials
NCERT solutions also help students to revise each concept in the subject before the exams begin. So, by going over the solutions, you can ensure that you answer all the questions that appear in the exams.
So, teachers should recommend to students to go through NCERT solutions for Chemistry so that they are thorough with all the concepts. The solutions are also a means by which you can answer questions that appear at the end of each chapter and assess your knowledge. Additionally, they also serve as reference materials and help clear any doubts that a student may have. So, instead of going to their teachers, students can refer to NCERT solutions and get all their doubts cleared.
Access NCERT Solutions For Class 11 Chemistry Chapter 11
Question 1. Discuss the pattern of variation in the oxidation states of
(i) B to Tl (ii) C to Pb.
Answer: (i) B to Tl
Common oxidation states are +1 and +3. The stability of +3 oxidation state decreases from B to Tl. +1 oxidation state increases from B to Tl.
(ii) C to Pb
The common oxidation states are +4 and +2. Stability of +4 oxidation state decreases from C to Pb.
Details can be seen from the text part.
Question 2. How can you explain higher stability of BCl3 as compared to TlCl3?
Answer: BCl3 is quite stable. Because there is absence of d- and f-electrons in boron three valence electrons (2s2 2px1) are there for bonding with chlorine atom. In Tl the valence s-electron (6s2) are experiencing maximum inert pair effect. Thus, only 6p1 electron is available for bonding. Therefore, BCl3 is stable but TlCl3 is comparatively unstable.
Question 3. Why does borontrifluori.de behave as a Lewis acid?
Answer: In BF3, central atom has only six electrons after sharing with the electrons of the F atoms. It is an electron-deficient compound and thus behaves as a Lewis acid.
Question 4. Consider the compounds, BCl3, and CCl4. How will they behave with water justify?
Answer: In BCl3, there is only six electrons in the valence shell of B atom. Thus, the octet is incomplete and it can accept a pair of electrons from water and hence BCl3 undergoes hydrolysis. Whereas, in CCl4, C atom has 8 electrons and its octet is complete. That’s why it has no tendency to react with water.
CCl4 + H20 —————> No reaction
Question 5. Is boric acid a protonic acid? Explain.
Answer: Boric acid is a Lewis acid, it is not a protonic acid.
Boric acid accepts electrons from hydroxyl ion of H2O molecule.
B (OH)3 + 2HOH ————> [B (OH)4]– + H3O+
Question 6. Explain what happens when boric acid is heated.
Answer: On heating boric acid above 370 K, it forms metabolic acid, HB02 which on further heating yields boric oxide B2O3.![]()
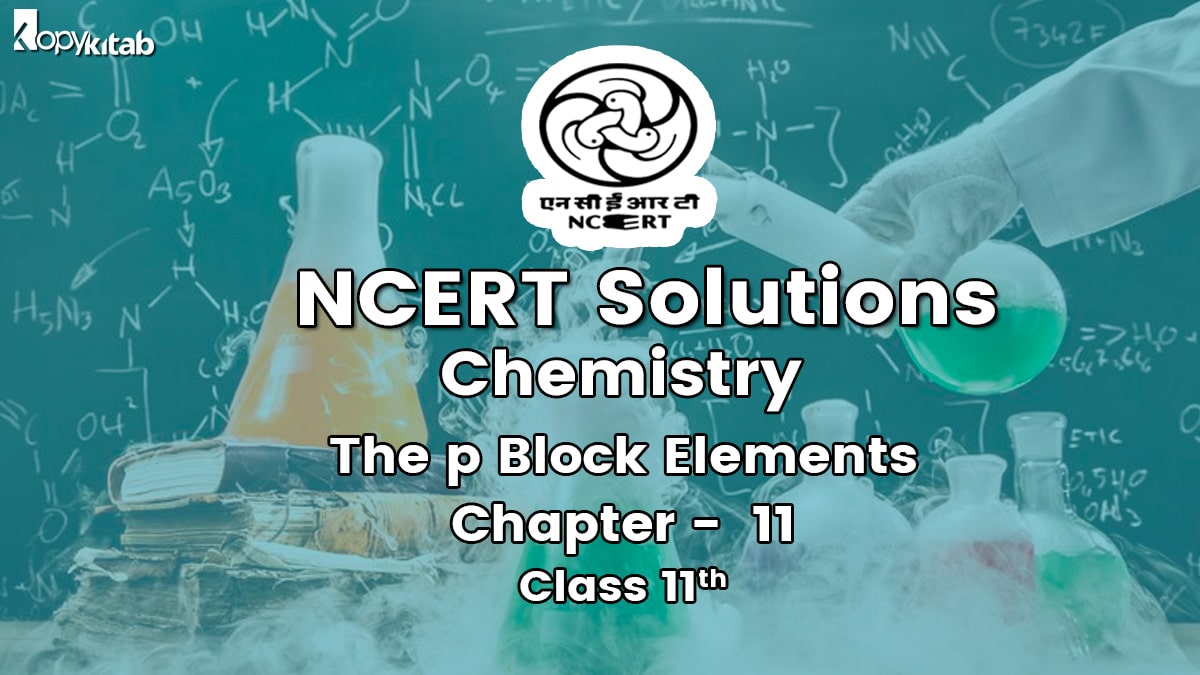
Question 7. Describe the shapes of BF3 and BH4–. Assign the hybridisation of boron in these species.
Answer: In BF3, boron is SP2 hybridized.
∴ shape of BF3 = planar.
In [BH4]–, boron is sp3 hybridized, thus the shape is tetrahedral.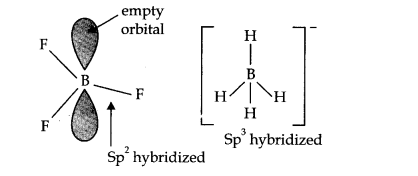
Question 8. Write reactions to justify amphoteric nature of aluminium.
Answer: Aluminium reacts with acid as well as base. This shows amphoteric nature of aluminium.
2Al(s) + 6HCl(dil.) ——> 2AlCl3(aq) + 3H2(g)
2Al(s) + 2NaOH(aq) + 6H2O(l) ———-> 2Na+ [Al(OH)4]–(aq) + 3H2(g)
Question 9. What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient species? Explain.
Answer: Electron deficient species are those in which the central atom in their molecule has the tendency to accept one or more electron pairs. They are also known as Lewis acid. BCl3 and SiCl4 both are electron deficient species.
Since, in BCl3, B atom has only six electrons. Therefore, it is an electron deficient compound.
In SiCl4 the central atom has 8 electrons but it can expand its covalency beyond 4 due to the presence of d-orbitals.
Thus, SiCl4 should also be considered as electron-deficient species.
Question 10. Write the resonance structure of CO32- and HCO3– .
Answer: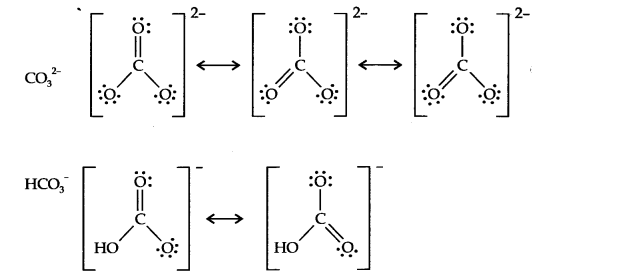
Question 11. What is the state of hybridisation of carbon in
(a) CO32- (b) diamond (c) graphite?
Answer: (a) CO32- (sp2) (b) Diamond (sp3) (c) Graphite (sp2)
Question 12. Explain the difference in properties of diamond and graphite on the basis of their structures.
Answer:
- Since diamond exists as a three dimensional network solid, it is the hardest substance known with high density and high melting point.
Whereas in graphite, any two successive layers are held together by weak forces of attraction. This makes graphite soft. - In graphite, carbon atom is sp2 hybridized whereas in diamond, carbon atom is sp3 hybridized.
- Unlike diamond, graphite is good conductor of heat and electricity.
Question 13.
- Rationalize the given statements and give chemical reactions:
- Lead (II) chloride reacts with Cl2 to give PbCl4 .
- Lead (IV) chloride is highly unstable towards heat.
- Lead is known not to form an iodide Pbl4.
Answer:
- PbCl2 + Cl2 ———> PbCl4.
This is because Pb can show +2 oxidation state more easily than +4 due to inert pair effect.
heat - PbCl4 ———> PbCl2 + Cl2
Because Pb2+ is more stable than Pb4+ due to inert pair effect. - Pbl4 does not exist because I- ion being a powerful reducing agent reduces Pb4+ ion to Pb2+ ion in solution.
- Pb4+ + 2I– ——-> Pb2+ + l2
Pb(IV) Pb(II)
Question 14. Suggest reason why the B-F bond lengths in BF3 (130 pm) and BF– (143 pm) differ.
Answer: In BF3 ‘B’ is sp2 hybridised and in BF4– ‘B’ is sp3 hybridised. Thus, the difference in bond length is due to the state of hybridisation.
Question 15. If B-Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
Answer: B-Cl bond has dipole moment because of polarity. In BCl3 since the molecule is symmetrical (planar). Thus the polarities cancel out.
Question 16. Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reason.
Answer: Since, anhydrous HF is covalent compound and weak acid due to high bond dissociation energy. AlF3 does not dissolve in HF.
Whereas NaF is ionic compound.
3NaF + AlF3 ———> Na3[AlF6]
Na3[AlF6] + 3BF3(g) ——-> AlF3 + 3Na+ [BF]–
Question 17. Suggest a reason as to why CO is poisonous.
Answer: CO reacts with haemoglobin to form carboxyhaemoglobin which can destroy the oxygen carrying capacity of haemoglobin and the man dies of suffocation.
Question 18. How is excessive content of C02 responsible for global warming?
Answer: Excess of C02 absorbs heat radiated by the earth. Some of it is dissipated into the atmosphere while the remaining part is radiated back to the earth. As a result, temperature of the earth increases. This is the cause of global warming.
Question 19. Explain structures of diborane and boric acid.
Answer: Boric acid contains planar BO33- ions which are linked together through hydrogen bonding shown in the fig.
Question 20. What happens when
(a) Borax is heated strongly
(b) Boric acid is added to water
(c) Aluminium is treated with dilute NaOH
(d) BF3 is reacted with ammonia?
Answer: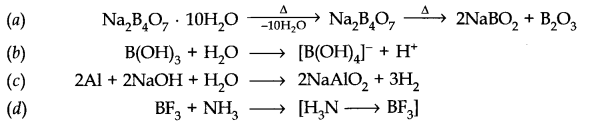
Question 21. Explain the following reactions.
(a) Silicon is heated with methyl chloride at high temperature in the presence of copper.
(b) Silicon dioxide is treated with hydrogen fluoride.
(c) CO is heated with ZnO.
(d) Hydrated alumina is treated with aqueous NaOH solution.
Answer: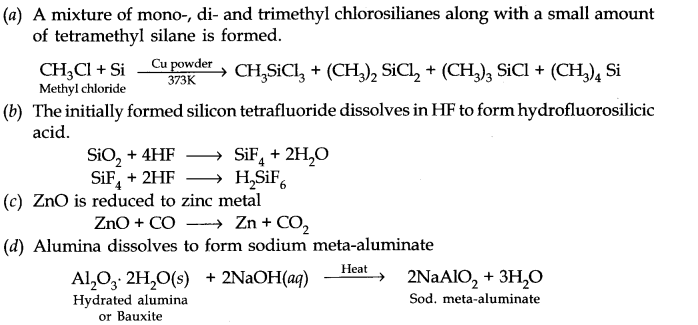
Question 22. Give reasons:
(i) Cone. HNO3 can be transported in aluminium container.
(ii) A mixture of dilute NaOH and aluminium pieces is used to open drain.
(iii) Graphite is used as lubricant.
(iv) Diamond is used as an abrasive.
(v) Aluminium alloys are used to make aircraft body.
(vi) Aluminium utensils should not be kept in water overnight.
(vii) Aluminium wire is used to make transmission cables.
Answer: (i) Al reacts with cone. HNO3 to form a very thin film of aluminium oxide on its surface which protects it from further reaction.
2Al(s) + 6HNO3(conc.) ———> Al2O3(s) + 6NO2(g) + 3H2O(l)
(ii) NaOH reacts with Al to evolve H2 gas. Thus the pressure of the gas produced can be used for clogged drains.
2Al(s) + 2NaOH(aq) + 2H2O(l) ——-> 2NaAlO2(aq) + 3H2(g)
(iii) Graphite has layered structure which are held by weak van der Waals forces. Thus, graphite cleaves easily between the layers, therefore it is very soft and slippery. That’s why it is used as lubricant.
(iv) Diamond is used as an-abrasive because it is an extremely hard substance.
(v) Alloys of aluminium, like duralumin, is used to make aircraft body due to some of its property like toughness, lightness and resistant to corrosion.
(vi) Generally, aluminium metal does not react with water quickly but, when it is kept overnight, it reacts slowly with water in presence of air.
2Al(s) + O2(g) + H2O(l) ——–> Al2O3(S) + H2(g)
a very small amount of (in ppm) Al3+ produced in the solution is injurious to health if the water is used for drinking purposes.
(vii) Aluminium is generally unaffected by air and moisture and it is also good conductor of electricity. That’s why it is used in transmission cables.
Question 23. Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon.
Answer: Because there is increase in atomic size on moving from carbon to silicon, the screening effect increases. Thus the force of attraction of nucleus for the valence electron decreases as compared to carbon. Thus the ionization enthalpy decreases from carbon to silicon.
Question 24. How would you explain the lower atomic radius of Ga as compared to Al?
Answer: Due to poor shielding effect of d-electrons in Ga, the electrons in gallium experience great force of attraction by nucleus as compared to Al.
Question 25. What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Answer: Allotropes: Allotropes are the different forms of an element which are having same chemical properties but different physical properties due to their structures.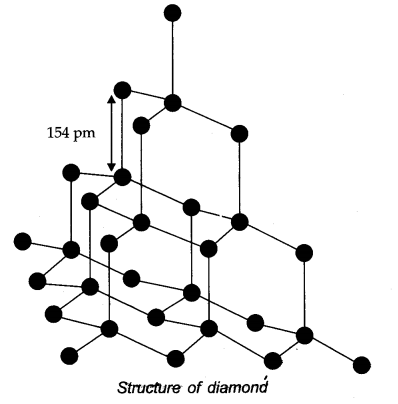
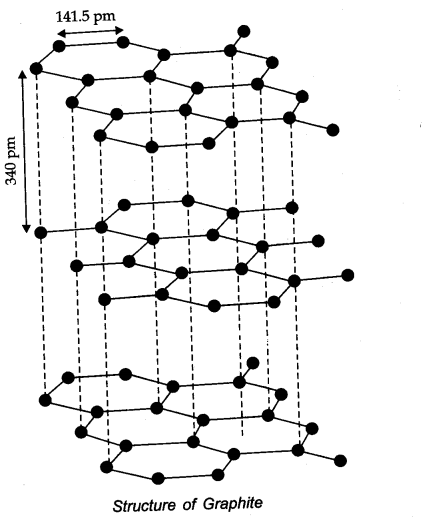
In diamond, carbon is SP3 -hybridized. Since, diamond is three dimensional network solid, it is hardest substance with high density whereas graphite has a layered structure.
The various layers are formed by van der Waals forces of attraction that’s why graphite is soft and slippery.
Question 26. (a) Classify the following oxides as neutral, acidic, basic or amphoteric
CO, B2O2, Si02, C02, Al2O3, PbO2, Tl2O3
(b) Write suitable equations to show their nature.
Answer: (a) Neutral — CO
Acidic — B2O2, Si02, C02 Basic — Tl2O3 Amphoteric — Tl2O3, PbO2
(b)-CO does not react with acid as well as base at room temperature.
Being acidic B2O3, Si02 and C02 reacts with alkalis to form salts.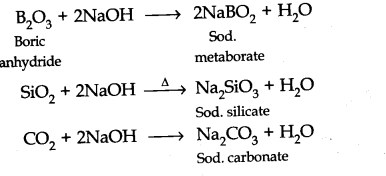
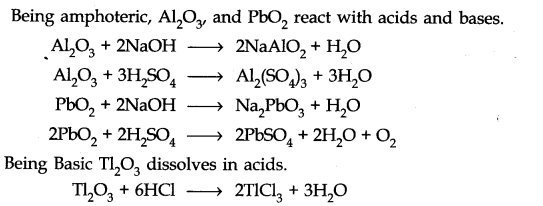
Question 27. In some of the reactions thallium resembles aluminium, whereas in others it resembles with group 1 metals. Support this statement by giving some evidences.
Answer: Tl shows both the oxidation state +1 and +3 due to inert pair effect. Tl forms basic oxide like group I elements. TlO2 is strongly basic.
Question 28. When metal X is treated with sodium hydroxide, a white precipitate (A) is obtained, which is soluble in excess of NaOH to give soluble complex (B). Compound (A) is soluble in dilute HCl to form compound (C).
The compound (A) when heated strongly gives (D), which is used to extract metal. Identify (X), (A), (B), (C) and (D). Write suitable equations to support their identities.
Answer:
Question 29. What do you understand by (a) inert pair effect (b) allotropy and (c) catenation?
Answer: (a) Inert pair effect: The pair of electron in the valence shell does not take part in bond formation is called inert pair effect.
(b)Allotropy: It is the property of the element by which an element can exist in two or more forms which have same chemical properties but different physical properties due to their structures.
(c)Catenation: The property to form chains or rings not only with single bonds but also with multiple bonds with itself is called catenation.
For example, carbon forms chains with (C-C) single bonds and also with multiple bonds (C = C or C = C).
Question 30. A certain salt X, gives the following results.
(i) Its aqueous solution is alkaline to litmus.
(ii) It swells up to a glassy material Y on strong heating.
(iii) When cone.H2SO4is added to a hot solution of X, white crystal of an acid Z separates out.
Answer:![]()
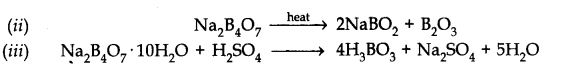
Question 31. Write balanced equations for:
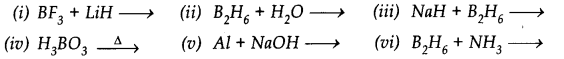
Answer: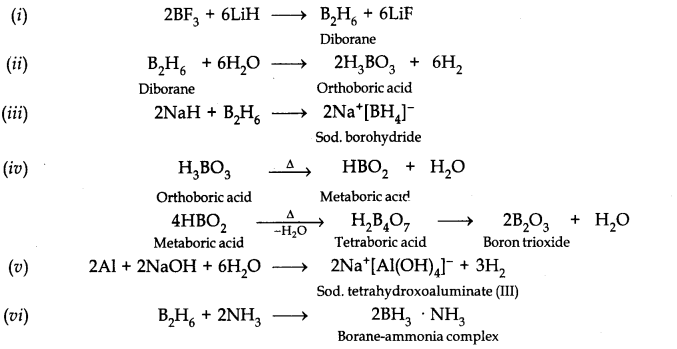
Question 32. Give one method for industrial preparation and one for laboratory preparation of CO and C02 each.
Answer: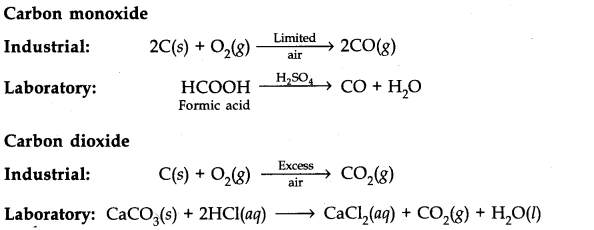
Question 33. An aqueous solution of borax is
(a) neutral (b) amphoteric (c) basic (d) acidic
Answer: Borax is a salt of a strong base (NaOH) and a weak acid (H3BO3), therefore, it is basic in nature, i.e., option (c) is correct.
Question 34. Boric acid is polymeric due to
(a) its acidic nature (b) the presence of hydrogen bonds
(c) its monobasic nature (d) its geometry
Answer: Boric acid is polymeric due to the presence of H-bonds. Therefore, option (b) is correct.
Question 35. The type of hybridisation of boron in diborane is
(a) sp (b) sp2(c) sp3(d) dsp2
Answer: In B2H6, B is sp3-hybridized. Therefore, option (c) is correct.
Question 36. Thermodynamically the most stable form of carbon is
(a)diamond (b) graphite (c) fullerenes (d) coal
Answer: Thermodynamically the most stable form of carbon is graphite, i.e., option (b) is correct.
Question 37. Elements of group 14
(a) exhibit oxidation state of +4 only (b) exhibit oxidation state of +2 and +4
(c) form M2-and M4+ ion (d) form M2+ and M4+ ions.
Answer: Due to inert pair effect, elements of group 14 exhibit oxidation states of +2 and +4. Thus, option (b) is correct.
We have covered the complete guide on CBSE NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements. Feel free to ask us any questions in the comment section below.
FAQ: NCERT Solutions for Class 11 Chemistry Chapter 11 The p-Block Elements
What are p-block elements in chemistry class 11?
P-Block elements: Contains, metals, non-metals, and metalloids. – Boron is a typical non-metal and the other members are metals.
What are the topics included in NCERT solutions Class 11 Chemistry Chapter 11?
You can refer to the above article to get the detailed topics taught in
NCERT solutions Class 11 Chemistry Chapter 11.
What are p-block elements called?
Collectively, these p-block elements are known as halogens.
How many metals are there in p-block?
There are 35 p-block elements, all of which are in p orbital with valence electrons.
can I download NCERT Solutions for Class 11 Chemistry Chapter 11 PDF free?
Yes, you can download NCERT Solutions for Class 11 Chemistry Chapter 11 PDF free.
cool, you have solved my all queries with this perfect solution. hoping these solutions are based on the latest syllabus of CBSE
Yes, it is based on the latest syllabus. Thank You So Much For Your Feedback