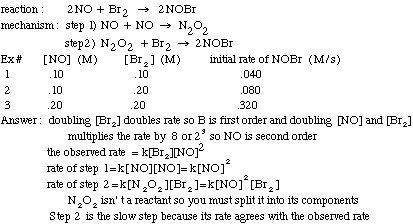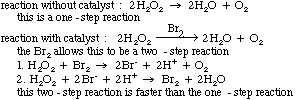Kinetics Notes
Kinetics is the area of chemistry concerned with reaction rates. The rate can be expressed as:
- collide
- have the right energy
- have the right geometry
To increase the rate, you must make the above more likely to occur. This is possible by changing other factors such as:
- increasing the surface area (of solids)-this allows for more collisions and gives more molecules the right geometry
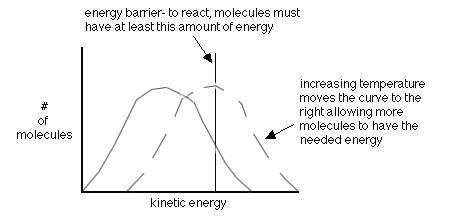
- increasing the temperature-this gives more molecules the right energy (also called the activation energy, Ea)
- increasing the concentration (of gases and solutions)-this allows for more collisions and more correct geometry
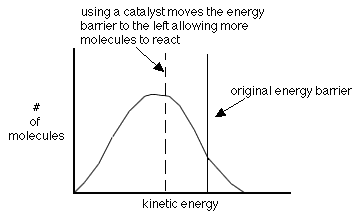
- using a catalyst-helps molecules achieve the correct geometry by providing a different way to react
The reaction rate can also be expressed by using a “rate law” and is written as follows:
[A], [B], etc. are the concentrations of the reactants
k is the rate constant or rate coefficient, a value dependent on temperature.
m,n, etc. are exponents that correspond to a, b, etc. The concentration is raised to the power of its coefficient in the balanced equation.
Reaction order is a topic that comes with reaction rates. If you have a reaction in that A, B, and C are possible reactants, then we can describe the order of the reaction following this chart:
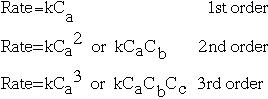
It is possible to determine the order of a reactant, and eventually the reaction rate, using initial rate information that includes the concentration of the reactants and the rate at that the product is formed. If you double the concentration of reactant X and the rate increases by 2a, then the order of reactant X is “a”. If you triple the concentration of reactant Y and the rate increases by 3b, then the order of Y is “b”. For example, if you have a reaction with one reactant, A, and you double [A] and the rate doubles, then the rate=k[A]1. If, instead, you double [A] and the rate quadruples, the rate=k[A]2. If you double [A] and the rate stays the same, then the rate=k[A]0.
To find the rate constant, k, using initial rate information, just plug in from the experiment one of the concentrations and rate into the rate law and solve. The units of k are trickier:
Ex: for 2nd order reaction, k=(M/s)/M2=M-1s-1
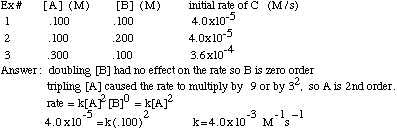
When dealing with reaction rates, it is sometimes important to know how to graph a straight line with the data you have. When graphing concentration versus time, there are two ways to graph a line. If you have a first order reaction, then the graph of ln[A] vs. time is a line. If you have a second order reaction, then the graph of 1/[A] vs. time produces a line.
A quantitative way to examine reaction rates is through Arrhenius Equation that states:
A is a constant related to the geometry needed
e is a constant, approximately 2.7281
Ea is the activation energy
R is the gas law constant, 8.314 J/mol-K
T is the temperature in kelvins
If it is a simple geometry to attain, A will be large. If a large Ea is needed then the exponent becomes more negative and therefore decreases k. If the temperature increases then the exponent becomes less negative and therefore increases k. A pop-up calculator is available to help practice using Arrhernius’ Equation to make calculations.
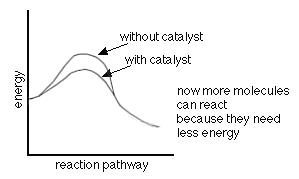
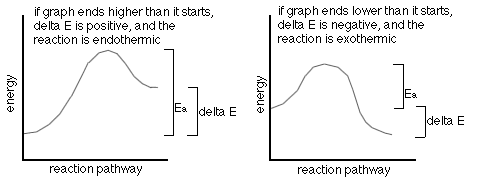
The following are two (2) energy profile graphs that help demonstrate energy changes during a reaction.
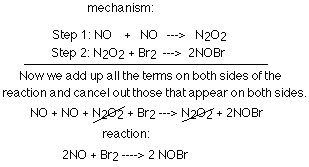
Example problem: Find the slow step of the following reaction mechanism