
NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry: Environmental Chemistry is an essential chapter for the class 11 exams. So, those students who aspire to come out on top must go through the chapter with the help of NCERT solutions. By doing so, you will get to know various concepts such as atmospheric pollution, causes and effects of global warming, greenhouse effect, and acid rain.
Additionally, you will be able to find out what causes the depletion of the ozone layer and the impact it has on the planet. Besides this, you will also study the causes and effects of water pollution and the standardization of drinking water across the world. Soil pollution, its causes, effects, and control, and the significance of green chemistry in everyday life are also among the topics students of class 11 would be studying.
NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry, has seven sections that explain various concepts related to the environment. The chapter addresses issues like environmental pollution and studies its causes and effects. The significant elements affected by pollution include water, soil, and the air around us. So, the chapter talks about how you can reduce pollution and a host of other concepts like the international standards for drinking water, waste management, and green chemistry.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry from below.
NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2020/09/chapter_14_environmental_-chemistry.pdf”, “#example1”);
What will you learn in NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry?
Environmental chemistry tells you about the origin, reactions, and the effects various chemicals have on the environment. The chapter focuses on studying the environment, which is a combination of social, biological, physical, and chemical entities.
Pollution
When harmful changes occur in the environment, it affects living beings such as plants and animals. The substances which cause these changes are known as a ‘pollutants.’ Pollutants exist in all states of matter, including solid, liquid, and gas. Humans produce pollutants when they set up industries or factories and release waste byproducts into the environment. Pollutants are degradable, meaning that the various natural processes that occur in the surroundings tend to break them down. However, other pollutants degrade at a slower rate and hence, remain in the surroundings for more extended periods, adversely affecting living creatures.
For instance, substances like plastic and metal are non-degradable and thus, lie around in the environment causing more damage.
Pollution is of two types. The first type, called Natural Pollution comes from natural sources such as volcanic eruptions. And the second type, also known as human-made pollution, is the result of human activities.
The atmosphere can also become polluted due to the release of oxides of carbon such as carbon monoxide.
Environment
The four different layers of the environment include the atmosphere, hydrosphere, lithosphere, and biosphere. The atmosphere is further sub-divided into five layers. They are the troposphere, stratosphere, mesosphere, thermosphere, and exosphere.
Types of Pollutants
While pollutants come in both biodegradable and non-biodegradable forms, they also form depending on their occurrence and existence. So, you have naturally occurred, and human-made pollutants also called primary and secondary pollutants.
Air Pollution
Nitrogen and Oxygen constitute the major components of the air that surrounds us. And other gases such as Argon and Carbon Dioxide make up the minor elements. Among these constituents, the oxides of Carbon, Nitrogen, and Sulphur are the primary causes of air pollution. Apart from these, other gases such as Ozone, CFC, different metals, hydrocarbons, smog, dust, and other pollutants also cause air pollution.
The significant effects of air pollution include acid rain, depletion of the ozone layer, and global warming.
Acid Rain
The acids of Nitrogen and Sulphur formed by the oxidation of the two elements is the primary cause of acid rain. Acid rains tend to occur more in industrial areas rather than in other areas and affect buildings, reducing their lifespans. They also have detrimental effects on the soil.
Depletion of the Ozone Layer
The release of chlorofluorocarbons or CFC is the primary cause for the depletion of the ozone layer that covers the earth and protects it from the harmful ultraviolet radiations. Ultraviolet radiations affect the photosynthesis process that takes place in plants.
Global Warming
Global warming is the effect of the release of gases like the oxides of carbon into the atmosphere. Due to industrialization and deforestation, the amount of carbon dioxide let out is increasing by the day. Global warming leads to the melting of ice caps, causing floods, rains, cyclones, and hurricanes, among other things. If not controlled, it is bound to affect agricultural production.
Benefits of Chapter 14 NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
There are hundreds of benefits of going over NCERT solutions for Chemistry, especially for a chapter like Environmental Chemistry. You will find such benefits in the section that follows.
1. Overall Understanding of the Class 11 Chemistry Chapter 14
By going through NCERT solutions, you will be thorough with the different concepts in the chapter.
2. Get to Know Probable Questions
Going over NCERT solutions will not only help you to become thorough with the concepts but also lets you know the kind of questions to expect in the exams.
3. Get Adequate Practice
By solving the exercise questions at the end of the chapter, you will get enough practice and confidence to face the exams.
4. Improves Speed and Accuracy
Solving exercise questions will help you to write answers in a faster and more effective way. NCERT Solutions for Class 11 Chemistry Chapter 14, teaches students the importance of the environment that surrounds us. It also tells you about the effects of pollution and global warming and how to control them. So, by going through the solutions and practicing writing answers to the questions at the end of the chapter, you can come out in flying colors.
Access NCERT Solutions For Class 11 Chemistry Chapter 14
Question 1. Define environmental chemistry?
Answer: Environmental Chemistry is the branch of science which deals with the chemical changes in the environment. It includes our surroundings such as air, water, soil, forest etc.
Question 2. Explain the tropospheric pollution in 100 words?
Answer: Tropospheric pollution occurs due to the presence of undesirable substance in air. These may be the solid or gaseous pollutants.
- Gaseous Air Pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
- Particulate Pollutants: These are dust, mist, fumes, and smog etc.
Question 3. Carbon monoxide gas is more dangerous than carbon dioxide gas. Why?
Answer: Carbon monoxide combines with haemoglobin to form a very stable compound known as carboxyhaemoglobin when its concentration in blood reaches 3-4%, the oxygen carrying capacity of the blood is greatly reduced.
This results into headache, nervousness and sometimes death of the person. On the other hand CO2 does not combine with haemoglobin and hence is less harmful than CO.
Question 4. Which gases are responsible for greenhouse effect? List some of them.
Answer: CO2 is mainly responsible for greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapors, CFCs and Ozone.
Question 5. Statues and monuments in India are affected by acid rain. How?
Answer: This is mainly due to the large number of industries and power plants in the nearby areas. Acid rain has vapors of sulphuric acid dissolved in it. When it comes in contact with various statues or monuments, the acid reacts chemically with calcium carbonate.
CaCO3 + H2SO4 ——–> CaSO4 + H2O + CO2
Question 6. What is smog? How is classical smog different from photochemical smog?
Answer: The word smog is a combination of smoke and fog. It is a type of air pollution that occurs in many cities throughout the world. Classical smog occurs in cool humid climate. It is also called as reducing smog.
Whereas photochemical smog occurs in warm and dry sunny climate. It has high concentration of oxidising agents and therefore, it is also called as oxidising smog.
Question 7. Write down the reactions involved during the formation of photochemical smog.
Answer: Mechanism of formation of photochemical smog: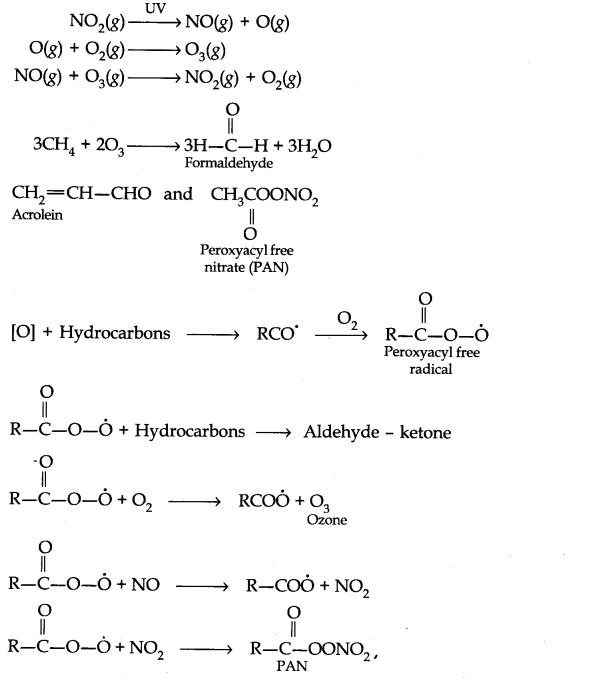
Question 8. What are the harmful effects of photochemical smog and how can they be controlled?
Answer: Harmful effects of photochemical smog:
- Their high concentration causes headache, chest pain and dryness of the throat.
- Ozone and PAN act as powerful eye irritants.
- Photochemical smog leads to cracking of rubber and extensive damage to plant life.
- It causes corrosion of metals, stones, building materials, and painted surface etc.
Control:
- Use of catalytic converter in automobiles prevents the release of nitrogen dioxide and hydrocarbons to the atmosphere.
- Pinus, junipers, quercus, pyrus etc. can metabolise nitrogen dioxide thus their plantation could help to some extent.
Question 9. What are the reactions involved for ozone layer depletion in the stratosphere?
Answer: The reaction can be shown as follows:
CF2Cl2(g) + UV ——-> Cl(g) + CF2Cl(g)
Cl(g) + O3(g) ———-> ClO (g) + O2(g)
ClO(g) + O(g) ———> Cl + O2(g)
Question 10. What do you mean by ozone hole? What are its consequences?
Answer: Depletion of ozone layer creates some sort of holes in the blanket of ozone which
surround us, this is known as ozone hole.
- With the depletion of the ozone layer, UV radiation filters into the troposphere which leads to aging of skin, cataract, sunburn, skin cancer etc.
- By killing many of the phytoplankton’s, it can damage the fish productivity.
- Evaporation rate increases through the surface and stomata of leaves which can decrease the moisture content of the soil.
Question 11. What are the major causes of water pollution? Explain.
Answer: Causes of water pollution:
- Pathogens: Pathogens include bacteria and other microorganisms that enter water from domestic sewage and animal excreta.
Human excreta contain bacteria such as Escherichia coli and Streptococcus faecalis ,
which cause gastrointestinal diseases. - Organic wastes: Organic wastes when added to water, as these are biodegradable, bacteria decomposes organic matter and consume dissolved oxygen in water. When the concentration of dissolved oxygen of water is below 6 ppm, the growth of fish gets inhibited. Breakdown of the organic wastes by anaerobic bacteria produces chemicals that have a foul smell and are harmful to human health.
- Chemical pollutants: Some inorganic chemicals as an industrial wastes dissolve in water like cadmium, mercury nickel etc. These metals are dangerous to humans and other animals. These metals can damage kidneys and central nervous system, lever etc. Petroleum products pollute many sources of water.
Question 12. What do you mean by Biochemical Oxygen Demand (BOD)?
Answer: The amount of oxygen required by bacteria to breakdown the organic matter present in a certain volume of a sample of water is called Biochemical Oxygen Demand (BOD).
Question 13. What are pesticides and herbicides? Explain giving examples.
Answer: Pesticides are the chemical compounds used in agriculture to control the damages caused by insects, rodents, weeds and various crop diseases.
Example: Aldrin, Dilldrin, B.H.C etc.
Herbicides: These are the chemicals used to control weeds.
Example: Triazines.
Question 14. What do you mean by green chemistry? How will it help in decreasing environmental pollution ?
Answer: Green chemistry is a way of thinking and is about utilizing the existing knowledge and principles of chemistry and other sciences to reduce the adverse effect of pollution.
For example:
- Automobile engines have been fitted with catalytic converters which prevent the release of the vapors of hydrocarbons and oxides of nitrogen into acrolein and peroxyacetyl nitrate.
- CO2 has replaced CFCs as blowing agents in the manufacture of polystyrene foam sheets.
Question 15. What would have happened if the greenhouse gases were totally missing in the earth’s atmosphere? Discuss.
Answer: The solar energy radiated back from the earth surface is absorbed by the green house gases. (CO2, CH4, O3, CFCs) are present near the earth’s surface.
They heat up the atmosphere near the earth’s surface and keep it warm.
As a result of these, there is growth of vegetation which supports the life. In the absence of this effect, there will be no life of both plant and animal on the surface of the earth.
Question 16. A large number offish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you find an abundance of phytoplankton. Suggest a reason for the fish kill.
Answer: Excessive phytoplankton (organic pollutants such as leaves, grass trash etc.) present in water are biodegradable. Bacteria decomposes these organic matters in water. During this process when large number of bacteria decomposes these organic matters, they-consume the dissolved oxygen in water. When the level of dissolved oxygen falls below 6 ppm the fish cannot survive.
Question 17. How can domestic waste be used as manure?
Answer: Domestic waste consists of biodegradable waste which can be converted into manure by suitable method.
Question 18. For your agricultural field or garden you have developed a compost producing pit. Discuss the process in the light of bad odor, flies and recycling of wastes for a good produce.
Answer: The compost producing pit should be kept covered so that flies cannot make entry into it and bad odor is minimized.
The waste materials which are non-biodegradable like glasses, plastic bags, polybags, must be handed over to the vendors who can send them to the recycling plants.
MORE QUESTIONS SOLVED
NCERT Solutions for Class 11 Chemistry Chapter 14 Very Short Answer Type Questions
Question 1. What do you mean by primary and secondary pollutants of the air?
Answer: Primary pollutants are those which after their formation remains as it was before e.g., NO. Secondary pollutants are formed as a reaction with primary pollutants e.g., PAN (peroxyacyl nitrates).
Question 2. What is the name of the compound formed when CO combines with blood?
Answer: Carboxyhaemoglobin.
Question 3. How are NO and NO2 formed in the atmosphere?
Answer: NO is formed due to the reaction between N2 and O2 during lightning or by the combustion of fossil fuels.NO is oxidised to form NO2.
Question 4. What is chlorosis?
Answer: Slowdown of process of formation of chlorophyll in plants with the presence of SO2 is called chlorosis.
Question 5. Which zone is known as ozonosphere?
Answer: Stratosphere.
Question 6. Which main gases is responsible for damage in ozone layer?
Answer: NO and CFCs
Question 7. What is the nature of classical smog?
Answer: Reducing.
Question 8. Name the acids which are responsible for acid rain?
Answer: H2SO4, HNO3 and HCl.
Question 9. What is BOD?
Answer: The amount of oxygen consumed by micro organism in decomposing organic wastes of sewage water is called BOD (Biochemical Oxygen Demand).
Question 10. What do you mean by viable and non-viable particulates?
Answer: Viable particulates are microorganisms like bacteria, fungi, moulds, algae etc. Non- viable particulates are formed by the disintegration of bigger size particles or by the condensation of water vapor, e.g., mist, smoke, fume and dust.
Question 11. What is siltation?
Answer: Mixing of soil or rock particles in water is called siltation.
Question 12. What is the composition of London Smog?
Answer: London Smog consists H2SO4 deposited on the particulates suspended in the atmosphere.
Question 13. List out the gases which are considered as major source of air pollution.
Answer: Carbon monoxide (CO), sulphur dioxide (SO2) and oxides of nitrogen (NO2).
Question 14. Why is acid rain considered as threat to Taj Mahal ?
Answer: Acids present in acid rain can react with marble (CaCO3) and damage the monument.
Question 15. Give one example of organic herbicide.
Answer: Triazines.
Question 16. What are pesticides ?
Answer: Pesticides are the substances used to kill unwanted pests. For example, DDT.
Question 17. What is PAN stands for?
Answer: It is peroxyacetyl nitrate.
Question 18. Give the examples of insecticides.
Answer: DDT, BHC.
Question 19. Which gas was mainly responsible for Bhopal gas tragedy?
Answer: Methyl isocyanate.
Question 20. What is meant by polar vortex?
Answer: A tight whirlpool of wind formed in the stratosphere which surrounds Antarctica is called polar vortex.
We have covered the complete guide on CBSE NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry. Feel free to ask us any questions in the comment section below.
FAQ: NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry
What are the key features of NCERT Solutions for Class 11 Chemistry Chapter 14?
NCERT Solutions for Class 11 Chemistry Chapter 14 provides solutions to all the exercise questions in the NCERT Class 11 Chemistry Chapter 14.
What are the Benefits of Chapter 14 NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry?
You can refer to the above article to get the Benefits of Chapter 14 NCERT Solutions for Class 11 Chemistry Chapter 14 Environmental Chemistry.
What is Global warming?
Global warming is the effect of the release of gases like the oxides of carbon into the atmosphere. Due to industrialization and deforestation, the amount of carbon dioxide let out is increasing by the day.
What will Chapter 14 Class 11 Chemistry teach?
Environmental chemistry tells you about the origin, reactions, and the effects various chemicals have on the environment. The chapter focuses on studying the environment, which is a combination of social, biological, physical, and chemical entities.
