 NCERT Solutions For Class 11 Chemistry Chapter 3: Getting ready with NCERT Solutions For Class 11 Chemistry Chapter 3 might seem easy at the beginning, however, it is not the case to be precise. Class 10 is just a pond compared to the syllabus prescribed in the 11th standard.
NCERT Solutions For Class 11 Chemistry Chapter 3: Getting ready with NCERT Solutions For Class 11 Chemistry Chapter 3 might seem easy at the beginning, however, it is not the case to be precise. Class 10 is just a pond compared to the syllabus prescribed in the 11th standard.
And if you aspire for a promising future then getting ready from NCERT Solutions For Class 11 Chemistry Chapter 3 should remain the ultimate goal.
But choosing the right path might confuse students from time to time. So, here we are presenting you NCERT Solutions For Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties, to begin with, your upcoming exam preparation.
Chemistry is unquestionably a crucial subject to get ready for a prospective future with science stream. Therefore understanding the topics loaded in the curriculum with ease is our utmost goal.
Therefore we offer you NCERT solutions for class 11 chemistry chapter 3 as robust assistance to study for the upcoming test.
NCERT Solutions For Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
Looking through the internet to get some solid study material to sit for chemistry exam preparation is not a hard task anymore, for we bring you NCERT Solutions For Class 11 Chemistry Chapter 3 pdf.
Download NCERT Solutions For Class 11 Chemistry Chapter 3 pdf from here and accommodate yourself with some effortless yet poignant course structure.
Download NCERT course material in your device like smartphone, desktop or laptop and start with our exam preparation anytime anywhere.
Moreover, the Hindi medium student grab your copy of NCERT solutions for class 11 chemistry chapter 3 pdf download in Hindi from here to have a rigorous understanding of the subject in no time.
You can download CBSE NCERT Solutions for Class 11 Chemistry Chapter 3 from below.
NCERT Solutions For Class 11 Chemistry Chapter 3
.pdfobject-container { height: 500px;}
.pdfobject { border: 1px solid #666; }
PDFObject.embed(“https://www.kopykitab.com/blog/wp-content/uploads/2021/07/chapter_3_classification_of_elements_and_periodicity.pdf”, “#example1”);
What will you learn in CBSE Class 11 Chemistry Chapter 3?
NCERT Solutions For Class 11 Chemistry Chapter 3 comes with one of the vital portions of the syllabus that acts pretty important for both class 11 and class 12.
Additionally, with strong preparation base, students will get tremendous help while appearing for JEE and NEET. The topics covered in this chapter are as follows-
- Genesis of periodic classification
- Modern periodic law and the present form of the periodic table
- Nomenclatures of elements with atomic number greater than 100
- Electronic configurations of elements and the periodic table
- The s-block, p-block, d-block, and f-block elements
- Metals, non-metals and metalloids
- Periodic trends in properties of elements
- Trends in physical properties
- Trends in chemical properties
- Trends in chemical reactivity
Types of questions that students get from this chapter are listed down-
- Question on the classification of elements
- Questions on periodic tables along with nomenclature of elements from Z>100
- Questions on quantum functions along with electronic configuration.
- Some more other important questions are on Atomic/ionic radii, ionization enthalpy, electron affinity, electronegativity and lastly reactivity of elements and more.
Subtopics From NCERT Solutions For Class 11 Chemistry Chapter 3
Subtopics of NCERT Solutions For Class 11 Chemistry Chapter 3 that students get to study in this chapter are,
- Why Do We Need To Classify Elements?
- Genesis Of Periodic Classification
- Modern Periodic Law And The Present Form Of The Periodic Table
- Nomenclature Of Elements With Atomic Numbers > 100
- Electronic Configurations Of Elements And The Periodic Table
- Electronic Configurations And Types Of Elements: 5-, P-, D-, F- Blocks Ex
- The S-block Elements
- The P-block Elements
- The D-block Elements (Transition Elements)
- The F-block Elements (Inner-transition Elements)
- Metals, Non-metals And Metalloids
- Periodic Trends In Properties Of Elements
- Trends in Physical Properties
- Periodic Trends In Chemical Properties
- Periodic Trends And Chemical Reactivity.
Benefits of NCERT Solutions For Class 11 Chemistry Chapter 3
- NCERT in collaboration with CBSE is doing great for the aspiring students out there. Additionally, the easy language base is effortlessly making the chapters get in fast and better for the students studying in 11th standard.
- These NCERT Solutions For Class 11 Chemistry Chapter 3 are a helping hand beside the classroom textbooks, so, students get the opportunity to wrap up the syllabus much before time.
- With quick completion of the chapters, students get several revision sessions to attempt which ultimately boosts their confidence for performing excellently during the actual exam.
- Moreover the more you go through the NCERT Solutions For Class 11 Chemistry Chapter 3, the better you get to understand your merit status, thus students get abundant scopes to work on their weakness on the subject, which again helps you a lot in performing brilliantly in your exam.
We have chalked down the essentials for you to invest your quality time in chemistry preparation. We hope you do great for your upcoming exam. But still, if you come across any uncertainties make sure to ask us since we are here to solve your problem out.
Access NCERT Solutions For Class 11 Chemistry Chapter 3
Question 1. What is the basic theme of organisation in the periodic table?
Answer: The basic theme of organisation of elements in the periodic table is to simplify and systematize the study of the properties of all the elements and millions of their compounds. This has made the study simple because the properties of elements are now studied in form of groups rather than individually.
Question 2. Which important property did Mendeleev use to classify the elements in this periodic table and did he stick to that?
Answer: Mendeleev used atomic weight as the basis of classification of elements in the periodic table. He did stick to it and classify elements into groups and periods.
Question 3. What is the basic difference in approach between Mendeleev’s Periodic Law and the Modem Periodic Law?
Answer: The basic difference in approach between Mendeleev’s Periodic Law and Modem Periodic Law is the change in basis of classification of elements from atomic weight to atomic number.
Question 4. On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Answer: The sixth period corresponds to sixth shell. The orbitals present in this shell are 6s, 4f, 5p, and 6d. The maximum number of electrons which can be present in these subshell is 2 + 14 + 6 + 10 = 32. Since the number of elements in a period corresponds to the number of electrons in the shells, therefore, sixth period should have a maximum of 32 elements.
Question 5. In terms of period and group where will you locate the element with z = 114?
Answer: Period – 7 and Group -14 Block-p.
Question 6. Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
Answer: The element is chlorine (Cl) with atomic number (Z) = 17.
Question 7. Which element do you think would have been named by
(i)Lawrence Berkeley Laboratory
(ii)Seaborg’s group?
Answer: (i) Lawrencium (Lr) with atomic number (z) = 103
(ii) Seaborgium (Sg) with atomic number (z) = 106.
Question 8. Why do elements in the same group have similar physical and chemical properties?
Answer: The elements in a group have same valence shell electronic configuration and hence have similar physical and chemical properties.
Question 9. What does atomic radius and ionic radius really mean to you?
Answer: Atomic radius. The distance from the centre of nucleus to the outermost shell o electrons in the atom of any element is called its atomic radius. It refers to both covalen or metallic radius depending on whether the element is a non-metal or a metal.
Ionic radius. The Ionic radii can be estimated by measuring the distances between cations and anions in ionic crystals.
Question 10. How do atomic radius vary in a period and in a group? How do you explain the variation?
Answer: Within a group Atomic radius increases down the group.
Reason. This is due to continuous increases in the number of electronic shells or orbit numbers in the structure of atoms of the elements down a group.
Variation across period.
Atomic Radii. From left to right across a period atomic radii generally decreases due
to increase in effective nuclear charge from left to right across a period.
Question 11. What do you understand by isoelectronic species? Name a species that will be iso electronic with each of the following atoms or ions.
(i) F–(ii) Ar (iii) Mg2+(iv) Rb+
Answer: Isoelectronic species are those species (atoms/ions) which have same number of
electrons. The isoelectronic species are:
(i)Na+ (iii) Na+
(ii)K+ (iv) Sr2+
Question 12. Consider the following species:
N3-, O2-, F–, Na+, Mg2+, Al3+
(a) What is common in them?
(b) Arrange them in order of increasing ionic radii?
Answer: (a) All of them are isoelectronic in nature and have 10 electrons each.
(b) In isoelectronic species, greater the nuclear charge, lesser will be the atomic or ionic radius.
Al3+ < Mg2+ < Na+ < F– < O2- < N3-
Question 13. Explain why cation are smaller and anions larger in radii than their parent atoms?
Answer: A cation is smaller than the parent atom because it has fewer electrons while its nuclear
charge remains the same. The size of anion will be larger than that of parent atom
because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge.
Question 14. What is the significance of the terms – isolated gaseous atom and ground state while defining the ionization enthalpy and electron gain enthalpy?[Hint: Requirements for comparison purposes]
Answer:
- Significance of term ‘isolated gaseous atom’. The atoms in the gaseous state are far separated in the sense that they do not have any mutual attractive and repulsive interactions.
- These are therefore regarded as isolated atoms. In this state the value of ionization enthalpy and electron gain enthalpy are not influenced by the presence of the other atoms.
- It is not possible to express these when the atoms are in the ; liquid or solid state due to the presence of inter atomic forces.
- Significance of ground state. Ground state of the atom represents the normal – energy state of an atom. It means electrons in a particular atom are in the lowest energy state and they neither lose nor gain electron.
- Both ionisation enthalpy and I electron gain enthalpy are generally expressed with respect to the ground state of an atom only.
Question 15. Energy of an electron in the ground state of the hydrogen atom is- 2.18 x 10-18 Calculate the ionization enthalpy of atomic hydrogen in terms of JMol-1.[Hint: Apply the idea of mole concept to derive the answer],
Answer: The ionisation enthalpy is for 1 mole atoms. Therefore, ground state energy of the , atoms may be expressed as E (ground state) = ( – 2.18 x 10-18 J) x(6.022 x 1023 mol-1)= -1.312 x 106 J mol-1
Ionisation enthalpy =E∞–E ground state
= 0-(-1.312 x 106mol-1)
= 1.312 x 106 J mol-1.
Question 16. Among the second period elements, the actual ionization enthalpies are in the order: Li <B< Be <C<0<KI<F< Ne
Explain why
(i) Be has higher ∆iH1than B ?
(ii) O has lower ∆iH1 than N and F?
Answer: (i) In case of Be (1s2 2s2) the outermost electron is present in 2s-orbital while in B (1s2 2s2 2p1) it is present in 2p-orbital. Since 2s – electrons are more strongly attracted by the nucleus than 2p-electrons, therefore, lesser amount of energy is required to knock out a 2p-electron than a 2s – electron. Consequently, At of Be is higher than that ∆iH1 of B.
(ii) The electronic configuration of
N7 = 1s2 2s2 2px1 2py1 2pz1
O8 =1s2 2s2 2px1 2py1 2pz1
We can see that in case of nitrogen 2p-orbitals are exactly half filled. Therefore, it is difficult to remove an electron from N than from O. As a result ∆iH1 of N is higher than that of O.
Question 17. How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
Answer: Electronic configuration of Na and Mg are
Na = 1s2 2s2 2p6 3s1
Mg = 1s2 2s2 2p6 3s2
First electron in both cases has to be removed from 3s-orbital but the nuclear charge of Na (+ 11) is lower than that of Mg (+ 12) therefore first ionization energy of sodium is lower than that of magnesium.
After the loss of first electron, the electronic configuration of
Na+ = 1s2 2s2 2p6
Mg+ = 1s2 2s2 2p6 3s1
Here electron is to be removed from inert (neon) gas configuration which is very stable and hence removal of second electron requires more energy in comparison to Mg.
Therefore, second ionization enthalpy of sodium is higher than that of magnesium.
Question 18. What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down the group?
Answer: Atomic size. With the increase in atomic size, the number of electron shells increase. Therefore, the force that binds the electrons with the nucleus decreases.
The ionization enthalpy thus decreases with the increase in atomic size. Screening or shielding effect of inner shell electron.
With the addition of new shells, the number of inner electron shells which shield the valence electrons increases. As a result, the force of attraction of the nucleus for the valence electrons further decreases and hence the ionization enthalpy decreases. ‘
Question 19. The first ionization enthalpy values (in kJ mol -1) of group 13 elements are:
B Al Ga In Tl
801 577 579 558 589
How would you explain this deviation from the general trend?
Answer: The decrease in ∆iH1 value from B to Al is due to the bigger size of Al.
In Ga there is 10 3d electrons which do not screen as is done by S and P electrons.
Therefore, there is an unexpected increase in the magnitude of effective nuclear charge resulting in increased ∆iH1 values. The same is with into Tl. The later has fourteen ∆f electrons with very poor shielding effect. This also increases, the effective nuclear charge thus the value of ∆iH1 increases.
Question 20. Which of the following pairs of elements would have a move negative electron gain enthalpy? (i) O or F (ii) F or Cl.
Answer: (i) O or F. Both O and F lie in 2nd period. As we move from O to F the atomic size decreases.
Due to smaller size of F nuclear charge increases.
Further, gain of one electron by
F —> F–
F~ ion has inert gas configuration, While the gain of one electron by
0->O–
gives CT ion which does not have stable inert gas configuration, consequently, the energy released is much higher in going from
F ->F–
than going from O —>O–
In other words electron gain enthalpy of F is much more negative than that of oxygen.
(ii) The negative electron gain enthalpy of Cl (∆ eg H = – 349 kj mol-1) is more than that of F (∆ eg H = – 328 kJ mol -1).
The reason for the deviation is due to the smaller size of F. Due to its small size, the electron repulsions in the relatively compact 2p-subshell are comparatively large and hence the attraction for incoming electron is less as in the case of Cl.
Question 21. Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer.
Answer: For oxygen atom:
O (g) + e– —> O– (g) (∆ eg H = – 141 kJ mol -1)
O– (g) + e– —> O 2- (g) (∆ eg H = + 780 kJ mol -1)
The first electron gain enthalpy of oxygen is negative because energy is released when a gaseous atom accepts an electron to form monovalent anion.
The second electron gain enthalpy is positive because energy is needed to overcome the force of repulsion between monovalent anion and second incoming electron.
Question 22. What is basic difference between the terms electron gain enthalpy and electro negativity?
Answer: Electron gain enthalpy refers to tendency of an isolated gaseous atom to accept an additional electron to form a negative ion. Whereas electronegativity refers to tendency of the atom of an element to attract shared pair of electrons towards it in a covalent bond.
23. How would you react to the statement that the electronegativity of on Pauling scale is 3.0 in all the nitrogen compounds?
Ans. On Pauling scale, the electronegativity of nitrogen, (3.0) indicates that it is sufficiently electronegative. But it is not correct to say that the electronegativity of nitrogen in all the compounds is 3.
It depends upon its state of hybridisation in a particular compound, greater the percentage of s-character, more will be the electronegativity of the element.
Thus, the electronegativity of nitrogen increases in moving from SP3 hybridised orbitals to SP hybridised orbitals i.e., as SP3 < SP2 < SP.
Question 24. Describe the theory associated with the radius of an atom as it:
(a) gains an electron (b) loses an electron ?
Answer:
- Gain of an electron leads to the formation of an anion. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among electrons and decrease in effective nuclear charge.
This the ionic radius of fluoride ion (F–) is 136 pm whereas atomic radius of Fluorine (F) is only 64 pm. - Loss of an electron from an atom results in the formation of a cation. A cation is smaller than its parent atom because it has fomer electrons while its nuclear charge remains the same.
- For example, The atomic radius of sodium (Na) is 186 pm and atomic radius of sodium ion (Na+) = 95 pm.
Question 25. Would you expect the first ionization enthalpies of two isotopes of the same element to be the same or different? Justify your answer.
Answer: Ionization enthalpy, among other things, depends upon the electronic configuration (number of electrons) and nuclear charge (number of protons). Since isotopes of an element have the same electronic configuration and same nuclear charge, they have same ionization enthalpy.
Question 26. What are major differences between metals and non-metals?
Answer: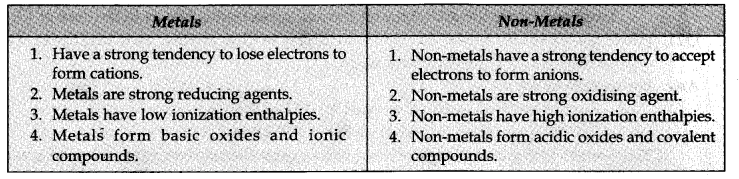
Question 27. Use periodic table to answer the following questions:
(a) Identify the element with five electrons in the outer subshell.
(b) Identify the element that would tend to lose two electrons.
(c) Identify the element that would tend to gain two electrons.
Answer: (a) Element belonging to nitrogen family (group 15) e.g., nitrogen.
(b) Element belonging to alkaline earth family (group 2) e.g., magnesium.
(c) Element belonging to oxygen family (group 16) e.g., oxygen.
Question 28. The increasing order of reactivity among group 1 elements is Li < Na < K < Rb < Cs whereas that of group 17 is F > Cl > Br > I. Explain?
Answer: The elements of Group I have only one electron in their respective valence shells and thus have a strong tendency to lose this electron. The tendency to lose electrons in turn, depends upon the ionization enthalpy.
Since the ionization enthalpy decreases down the group therefore, the reactivity of group 1 elements increases in the same order Li < Na < K < Rb < Cs.
In contrast, the elements of group 17 have seven electrons in their respective valence shells and thus have strong tendency to accept one more electron to make stable configuration. It is linked with electron gain enthalpy and electronegativity.
Since both of them decreases down the group, the reactivity therefore decreases.
Question 29. Write the general electronic configuration of s– p– d–, and f-block elements?
Answer: (i) s-Block elements: ns 1-2 where n = 2 – 7.
(ii) p-Block elements: ns2 np1-6 where n = 2-6.
(iii) d-Block elements:(n – 1) d1-10 ns 0-2 where n = 4-7.
(iv) f-Block elements: (n – 2) f0-14 (n -1) d0-1 ns2where n = 6 – 7.
Question 30. Assign the position of the element having outer electronic configuration,
(i) ns2 np4 for n = 3 (ii) (n – 1) d2 ns2 for n = 4 and (iii) (n – 2) f7 (n – 1) d1 ns2 for n = 6 in the periodic table?
Answer: (i) n = 3
Thus element belong to 3rd period, p-block element.
Since the valence shell contains = 6 electrons, group No = 10 + 6 = 16 configuration =1s2 2s2 2p6 3s2 3p4 element name is sulphur.
(ii) n = 4
Means element belongs to 4th period belongs to group 4 as in the valence shell (2 + 2) = 4 electrons.
Electronic configuration.=1s2 2s2 2p6 3s2 3p6 3d2 4s2, and the element name is Titanium (T i).
(iii) n = 6
” Means the element belongs to 6th period. Last electron goes to the f-orbital, element is from f-block.
group = 3
The element is gadolinium (z = 64)
Complete electronic configuration =[Xe] 4 f7 5d1 6s2.
Question 31.


Which of the above elements is likely to be:
(a) the least reactive element (b) the most reactive metal
(c) the most reactive non-metal (d) the least reactive non-metal
(e) the metal which can form a stable binary halide of the formula MX2(X = halogen)
(f) the metal which can form a predominantly stable covalent halide of the formula MX (X = halogen)?
Answer: (a) The element V has highest first ionization enthalpy (∆ iH1) and positive electron gain enthalpy (∆eg H) and hence it is the least reactive element. Since inert gases have positive eg H, therefore, the element-V must be an inert gas. The values of ∆ i H1, ∆ iH2 and ∆eg H match that of He.
(b) The element II which has the least first ionization enthalpy (∆ i H1) and a low negative electron gain enthalpy (∆eg H) is the most reactive metal. The values of ∆ i H1, ∆ iH2 and ∆eg H match that of K (potassium).
(c) The element III which has high first ionization enthalpy (∆ iH1) and a very high negative electron gain enthalpy (∆eg H) is the most reactive non-metal. The values of ∆ i H1, ∆ iH2 and ∆eg H match that of F (fluorine).
(d) The element IV has a high negative electron gain enthalpy (∆eg H ) but not so high first ionization enthalpy (∆eg H). Therefore, it is the least reactive non-metal. The values of ∆ i H1, ∆ iH2 and ∆eg H match that of I (Iodine).
(e) The element VI has low first ionization enthalpy (∆ iH1) but higher than that of alkali metals. Therefore, it appears that the element is an alkaline earth metal and hence will form binary halide of the formula MX2(where X = halogen). The values of ∆ i H1, ∆ iH2 and ∆eg H match that of Mg (magnesium).
(f) The element I has low first ionization (∆ iH1) but a very high second ionization enthalpy (∆ iH2), therefore, it must be an alkali metal. Since the metal forms a predominarly stable covalent halide of the formula MX (X = halogen), therefore, the alkali metal must be least reactive. The values of ∆ i H1, ∆ iH2 and ∆eg H match that of Li (lithium).
Question 32. Predict the formulas of the stable binary compounds that would be formed by the combination of the following pairs of elements:
(a) Lithium and oxygen(b) Magnesium and nitrogen
(c) Aluminium and iodine(d) Silicon and oxygen
(e) Phosphorous pentafluoride (f) Element 71 and fluorine.
Answer: (a) Li02(Lithium oxide) (b) Mg3N2(Magnesium nitride)
(c) AlI3(Aluminium iodide) (d) Si02 (Silicon dioxide)
(e) Phosphorous and fluorine (f) Z = 71
The element is Lutenium (Lu). Electronic configuration[Xe] 4 f7 5d1 6s2.with fluorine it will form a binary compound = Lu F3.
Question 33. In the modem periodic table, the period indicates the value of
(a)atomic number (b) mass number (c) principal quantum number (d) azimuthal quantum number?
Answer: In the modern periodic table, each period begins with the filling of a new shell. Therefore, the period indicates the value of principal quantum number. Thus, option (c) is correct.
Question 34. Which of the following statements related to the modem periodic table is incorrect?
(a) The p-block has six columns, because a maximum of 6 electrons can occupy all the orbitals in a p-subshell.
(b) The d-block has 8 columns, because a maximum of 8 electrons can occupy all the orbitals in a d-subshell.
(c) Each block contains a number of columns equal to the number of electrons that can occupy that subshell.
(d) The block indicates value of azimuthal quantum number (l)for the last subshell that received electrons in building up the electronic configuration.
Answer: Statement (b) is incorrect.
Question 35. Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons.
Answer: (c) Nuclear mass.
Question 36. The size of isoelectronic species-F–, Ne and Na+ is affected by
(a) nuclear charge (Z)
(b) valence principal quantum number (n)
(c) electron-electron interaction in the outer orbitals
(d) none of the factors because their size is the same
Answer: (a) Nuclear charge (Z).
Question 37. Which of the following statements is incorrect in relation to ionization enthalpy?
(a)ionization enthalpy increases for each successive electron.
(b)The greatest increase in ionization enthalpy is experienced on removal of electrons from core noble gas configuration.
(c)End of valence electrons is marked by a big jump in ionization enthalpy.
(d)Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
Answer: (d) is incorrect.
Question 38. Considering the elements B, Al, Mg and K, the correct order of their metallic character is:(a) B> Al> Mg > K(b) Al> Mg > B> K (c) Mg > Al> K> B (d) K> Mg > Al> B
Answer: In a period, metallic character decreases as we move from left to right. Therefore, metallic character of K, Mg and Al decreases in the order: K > Mg > Al. However, within a group, the metallic character, increases from top to bottom.
Thus, Al is more metallic than B. Therefore, the correct sequence of decreasing metallic character is: K > Mg > Al > B, i.e., option (d) is correct.
Question 39. Considering the elements B, C, N, F and Si, the correct order of their non-metallic character is: (a) B>C>Si>N>F (b) Si>C>B>N>F (c) F>N>C>B>Si (d) F>N>C>Si>B
Answer: In a period, the non-metallic character increases from left to right. Thus, among B, C, N and F, non-metallic character decreases in the order: F > N > C > B. However, within a group, non-metallic character decreases from top to bottom.
Thus, C is more non-metallic than Si. Therefore, the correct sequence of decreasing non-metallic character is: F > N > C > B > Si, i.e., option (c) is correct.
Question 40. Considering the elements F, Cl, O and N, the correct order of their chemical reactivity in terms of oxidising property is:
(a) F > Cl> O > N (b) F > O > Cl> N (c) Cl> F > O > N (d) O > F > N > Cl
Answer: Within a period, the oxidising character increases from left to right. Therefore, among F, O and N, oxidising power decreases in the order: F > O > N. However, within a group, oxidising power decreases from top to bottom. Thus, F is a stronger oxidising agent than Cl.
Further because O is more electronegative than Cl, therefore, O is a stronger oxidising agent than Cl. Thus, overall decreasing order of oxidising power is: F > O > Cl > N, i.e., option (b) is correct.
MORE QUESTIONS SOLVED
I. Very Short Answer Type Questions
Question 1. State the Modem Periodic Law.
Answer: Modem Periodic Law states that physical and chemical properties of the elements are a periodic function of their atomic numbers.
Question 2. Why is ionization enthalpy of nitrogen greater than that of oxygen?
Answer: Nitrogen has exactly half filled p-orbitals.
Question 3. Why are electron gain enthalpies of Be and Mg positive?
Answer: They have fully filled s-orbitals and hence have no tendency to accept an additional electron. That’s why energy is needed if an extra electron is to be added. Therefore, electron gain enthalpies of Be and Mg are positive.
Question 4. Give four examples of species which are isoelectronic with ca2+.
Answer: Ar, K+, CT, S2-, or P3- are isoelectronic with ca2+.
Question 5. Which two elements of the following belong to the same period?
Al, Si, Ba and O
Answer: Al and Si.
Question 6. Explain why chlorine can be converted into chloride ion more easily as compared to fluoride ion from fluorine ?
Answer: Electron gain enthalpy of Cl is more negative than that of F.
Question 7. What are horizontal rows and vertical columns of the periodic table called?
Answer: Horizontal rows are called periods and vertical columns are called groups.
Question 8. Which has a larger radius?
(i)Mg or Ca (ii) S or Cl
Answer: (i) Ca (ii) S.
Question 9. What are representative elements?
Answer: The elements of group 1 (alkali metals), group 2,(alkaline earth metals) and group 13 to 17 constitute the representative elements. They are elements of s-block and p-block.
Question 10. Give general electronic configuration off-block elements?
Answer: General electronic configuration of f-block elements =(n – 2) f1-14 (n -1) d0-1 ns2.
Question 11. What are inner transition metals? Why are they called rare earth metals?
Answer: Lanthanoids (the fourteen elements after Lanthanum) and actinides (the fourteen elements after actinium) are called inner transition elements.
Question 12. Define ionisation enthalpy.
Answer: It is the energy required to remove an electron from an isolated gaseous atom in its ground state. M (g) + I.E. àM+ (g) + e–
Question 13. The electronic configuration of an element is Is 2s 2p 3s 3p 4s . Locate the element in the periodic table.
Answer:
- As the principal quantum number for the valence shell is 4, the element is present in the 4th period.
- Since the last electron has been filled in 4s sub-shell (or orbital), the element belongs to s-block.
- As there is only one electron in the valence s-sub-shell, the element is present in group I.
II. Short Answer Type Questions
Question 1. What is the cause of periodicity in properties of the elements? Explain with two examples.
Answer: The cause of periodicity in properties is the repetition of similar outer electronic configuration after certain regular intervals.
For example, all the elements of group LA i.e., alkali metals, have similar outer electronic configuration as ns1.
Where n refer to the number of outermost principal shell.
In a similar manner all the halogens i.e., elements of group VILA have similar other electronic configuration i.e., ns2 ns5 and hence possess similar properties.
Question 2. Show by a chemical reaction with water that Na20 is a basic oxide and Cl207 is an acidic oxide.
Answer: Na2 0reacts with water to form sodium oxide which turns red litmus blue.
Na20 +H20——-> 2NaOH
Sod.oxide Sod.hydroxide
Therefore, Na20 is a basic oxide
In contrast,Cl207 reacts with water to form perchloric acid which turns blue litmus red.
Cl207 +H20——–>2HClO4
perchloric acid
Therefore, Cl207 is an acidic oxide.
Question 3. What do you understand by ‘Representative elements’? Name the groups whose elements are called representative elements.
Answer: The elements of s and p-block are collectively called representative or main group elements. These include elements of group I (alkali metals), group 2 (alkaline earth metals). .
Question 4. Name different blocks of elements in the periodic table. Give general electronic configuration of each block.
Answer: Elements in the long form of the periodic table have been divided into four blocks i.e., s, p, d and f. This division is based upon the name of the orbital which receives the last electron.
General electronic configuration of s-block elements: ns1 – 2 where n = 2 – 7
p-block elements: ns2 np1- 6 where n = 2 – 6
d-block elements: (n -1) d1 -10 ns0- 2 where n = 4 – 7
f-block elements: (n – 2)f0-14(n -1) d0-1 ns2 where n = 6 – 7
Question 5. Elements A, B, C and D Iwoe atomic numbers 12,19, 29, and 36 respectively. On the basis of electronic configuration, write to which group of the periodic table each element belongs.
Answer: Electronic configuration of A (Z = 12)
=1s2 2s2 2p6 3s2
period = 3, Element’s name = Mg block = s, Group = II Electronic configuration of B (Z = 19)
Element’s name = K (potassium)
=1s2 2s2 2p6 3s2 3p6 4s1 n = 4, period = 4 Block = s, Group = I Electronic configuration of C (Z = 29)
=1s2 2s2 2p6 3s2 3p6 3d10 4s1 n – 4, period = 4 Block = d Electronic configuration of D (Z = 36)
=1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 period = 4 Block = p-Block group = 18
Question 6. Define the term ionization enthalpy? How does it vary along a period and along a group?
Answer: Ionization Enthalpy. The minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom so as to convert it into a gaseous cation is called its ionization enthalpy or energy. It is represented by A; H.
This process may be represented as M (g) + ∆iH -> M+ (g) + e–(g)
where M (g) is isolated gaseous atom.
M+ (g) is the resultant cation (a position ion)
Variation along a period. Moving from left to right in a period, the ionization enthalpy increases with atomic number.
Variation within a group. The ionization enthalpies keep on decreasing regularly as we move down a group from one element to the other.
Question 7. Discuss briefly the various factors on which ionization enthalpy depends.
Answer:
- Atomic size. With the increase in the atomic size, the number of electron shells
increases. Therefore, the force that binds the electrons with the nucleus decreases. Thus, the ionization enthalpy decreases with increase in atomic size. - Nuclear charge. As the magnitude of the positive charge on the nucleus of an atom increases, the attraction with the electrons also increases. Therefore, the ionization enthalpy increases with the increase in the magnitude of the nuclear charge.
- Screening or shielding effect. Greater the magnitude of the screening effect, less will be the value of ionization enthalpy or potential.
Question 8. What are Dobereiner triads? Name two such triads.
Answer: Dobereiner arranged certain elements with similar properties in groups of three in such a way that the atomic mass of the middle element was nearly the same as the average atomic masses of the first and third elements.
For example:
Triad: lithium sodium potassium
Atomic mass: 7 23 39
Atomic mass of Na =(39+7)/2= 23
Triad: Chlorine Bromine Iodine
Atomic mass: 35.5 80 127
Atomic mass of Br =127 + 35.5/2 = 81.25
Question 9. Give the electronic configuration of the transition elements. Write their four important characteristics.
Answer: The d-block elements are known as transition elements.
Electronic configuration =(n – 1) d1-10 ns1 -2
Characteristics of d-block elements:
- They show variable oxidation states.
- Their compounds are generally paramagnetic in nature.
- Most of the transition elements form coloured compounds.
- They are all metals with high melting and boiling points.
Question 10. What is screening or shielding effect? How does it influence the ionization enthalpy ?
Answer: In a multielectron atom, the electrons present in the inner shells shield the electrons in the valence shell from the attraction of the nucleus or they act as a screen between the nucleus and these electrons.
This is known as shedding effect or screening effect. As the screening effect increases, the effective nuclear charge decreases. Consequently, the force of attraction by the nucleus for the valence shell electrons decreases and hence the ionization enthalpy decreases.
Question 11. Define electron gain enthalpy. What are its units?
Answer: The energy which is released by an atom in gaining an electron from outside atom or ion to form negative ion (or anion) is called electron gain enthalpy (∆eg H).
Unit of electron gain enthalpy is kJ/ mol.
In some cases, like in noble gas, atoms do not have any attraction to gain an electron. In that case energy has to be supplied.
For example,
Ne (g) + e– —> Ne– (g)
∆eg H = + 116 kJ mol -1
III. Long Answer Type Questions
Question 1. Discuss the main features of long form of the periodic table. What are the advantages of long . form of periodic table?
Answer: Main features of long form of periodic table:
- Groups. The vertical columns in the periodic table are known as groups. There are 18 groups in the long form of periodic table.
Each group having the same electronic configuration in the outermost shell. - Periods. There are 7 periods in the long form of periodic table.
It is denoted by n which means highest principal quantum number. - Lanthanoids. Group of 14 elements in the sixth period. They are placed after Lanthanum.
- Actinides. Group of 14 elements in the seventh period after actinium. Both Lanthanoids and actinoids are placed in separate panel at the bottom of the periodic table.
Advantages of long form of periodic table:
- It gives a suitable link between the position of element and its electronic configuration.
- On the basis of atomic numbers it easier to remember all the elements.
- The elements in the same group have similar properties due to their outer-most (valence shell) configuration. Thus it gives is a logical classification.
- Justified positions are provided to transition and inner transition elements. ‘
- It makes the study of elements systematic and simple.
Question 2. Discuss the main characteristics of four blocks of elements in the periodic table? Give their general electronic configuration.
Answer: s-block elements:
- They are highly reactive elements and thus occurs in combined state. On moving down the group their reactivity increases.
- They have good reducing characters.
- They generally form electropositive ion by losing 1 or 2 electrons, that’s why they are electro positive in nature.
- They are good conductors of heat and electricity.
p-block elements:
- Most of the p-block elements show variable oxidation states.
- They include both metals and non-metals.
- They are generally covalent in nature.
- As move from left to right the non-metallic character of the element increases.
- On moving down the group metallic character increases.
d-block elements:
- d-block elements show variable oxidation states.
- They are generally paramagnetic in nature.
- Their compounds are generally coloured. Those which form complex compounds.
- Most of the elements and their compounds acts as catalyst.
f-block elements:
- They are generally heavy metals having high melting and boiling points.
- Their compounds are generally coloured.
- Variable oxidation states are generally shown by these elements.
- Most of Activities are radioactive.
General electronic configuration:
s-block —ns1-2
p-block —ns2 np1- 6
d-block —(n -1) d1 -10 ns0-2
f-block —(n – 2)f0-14 (n -1) d0- 1 ns2
Question 3. Define electron gain enthalpy. What are its units? Discuss the factors which influence the electron gain enthalpy.
Answer: Electron gain enthalpy is the energy released when an isolated gaseous atom is converted into a negative ion by adding an extra electron.
Electron gain enthalpy is denoted by the sign ∆eg H.
The process may be represented by
M(g) + e– ———————>M– (g)
neutral gaseous atom anion
∆ H=∆eg H
electron gain enthalpy is negative or positive it depends upon the nature of the element. For example. For halogens it is highly negative, because they can acquire the noble gas configuration by accepting an extra electron.
In contrast. For noble gases have positive electron gain enthalpy because energy has to be supplied to the element.
Factors on which electron gain enthalpy depends:
- Atomic size. As the size of an atom increases, the distance between its nucleus and the incoming electron also increases.
- Therefore, the force of attraction between the nucleus and the incoming electron decreases and hence the electron gain enthalpy becomes less negative.
- Nuclear charge. As the nuclear charge increases force of attraction for the incoming electron increases and thus electron gain enthalpy becomes more negative.
- Symmetry of electronic configuration. Elements having symmetrical configuration (Either half filled or fully filled orbitals in the same sub shell) having no attraction for electron because by accepting electron their configuration becomes less stable.
- In that case energy has to be supplied to accept electron. Thus electron gain enthalpy will be positive.
Question 4. Discuss the factors that influence the magnitude of ionization enthalpy. What are the general trends of variation of ionization enthalpy in the periodic table? Explain.
Answer: Factors affecting Ionization enthalpy.
- Atomic size. With the increase in atomic size, the number of electron shells increases and thus the force of attraction between the electrons and the nucleus decreases. Therefore the ionization enthalpy decreases.
- Nuclear charge. As the nuclear charge increases the attraction for the electron also increases that’s why ionization enthalpy increases.
- Screening or shielding effect. In a multi-electron atom, the electron present in the inner shells shield the electrons in the valence shell as a result these electrons
experience less attraction from the nucleus. - This leads to lesser ionization enthalpy.
- Variation along a period. On moving from left to right in a period the nuclear charge increases and the atomic size decreases as a result ionization enthalpies are expected to increase.
- Variation within a group. On moving down the group as the atomic size of the elements increases that’s why ionization enthalpy decreases down the group.
Question 5. (a) How does atomic radius vary in group in the periodic table?
(b) Explain
(i) Radius of cation is less than that of the atom.
(ii) Radius of anion is more than that of the atom.
(iii) In iso-electronic ion, the ionic radii decreases with increase in atomic number.
Answer: (a) Variation of atomic radius in a group:
On moving down the group there is an increase in the principal quantum number and therefore no. of electron shells increases and thus the atomic size increases. Thus the atomic radii of the element increases.
(b) (i) Radius of cation is less than that of the atom:
Since the cation is formed by losing of one or more electrons.
For example,
Na —> Na++ e–
Thus the radius of Na+ will be less than the Na.
(ii) Radius of anion is more than that of the atom.
Since the anion is formed by gaining one or more electron. Therefore, the atomic radius is larger than the corresponding atom.
(iii) In iso-electronic ions, atoms have same number of electrons but different magnitude of nuclear charges. As the nuclear charge increases ionic radius decreases.
For example. N3-, O2-, F– have same No. of electrons = 10 but different ionic radii = 171, 140, 136 respectively.
IV. Multiple Choice Questions
Question 1. The highest ionization energy is exhibited by
(a) halogens (b) alkaline earth metals
(c) transition metals (d) noble gases
Question 2. Which of the following oxides is neutral?
(a) Sn02 (b) CO (c) Al203 (d) Na20
Question 3. Which of the following is arranged in order of increasing radius?
(a) K+ (aq) < Na+ (aq) < Li+ (aq) (b) K+ (aq) > Na+ (aq) > Zn2+ (aq)
(c) K+ (aq) > Li+ (aq) > Na+ (aq) (d) Li+ (aq) < Na+ (aq) < K+ (aq)
Question 4. What is the electronic configuration of the elements of group 14?
(a) ns2 np4 (b) ns2 np6 (c) ns2 np2 (d) ns2
Question 5. Among the following elements, which has the least electron affinity?
(a) Phosphorous (b) Oxygen (c) Sulphur (d) Nitrogen
Question 6. In halogens, which of the following, increases from iodine to fluorine?
(a) Bond length (b) Electronegativity
(c) The ionization energy of the element (d) Oxidizing power
Question 7. Diagonal relationships are shown by
(a) Be and A1 (b) Mg and A1 (c) Li and Mg (d) B and P
Question 8. Which of the following species are not known?
(a) AgOH (b) PbI4 (c) PI5 (d) SH6
(e) All of the above
Question 9. Which one of the following is isoelectronic with Ne?
(a) N3- (b) Mg2+ (c) Al3+ (d)all of the above
Question 10.Which element has smallest size?
(a) B (b) N (c) Al (d) P
Answer: 1. (b) 2. (b) 3. (d) 4. (c) 5. (d)
6. (b) (c) and (d)7. (a) and (c)8. (e)9. (d) 10. (b)
V. Hots Questions
Question 1. Arrange the following as stated: (i) N2, 02, F2, Cl2(Increasing order of bond dissociation energy) (ii) F, Cl, Br, I (Increasing order of electron gain enthalpy) (iii) F2, N2, Cl2, O2(Increasing order of bond length).
Answer: (i) F2 < Cl2 < 02 < N2
(ii) I < Br < F < Cl
(iii) N2 < 02 < F2 < Cl2
Question 2. The first ionisation enthalpy of magnesium is higher than that of sodium. On the other hand, the second ionisation enthalpy of sodium is very much higher than that of magnesium. Explain.
Answer: The 1st ionisation enthalpy of magnesium is higher than that of Na due to higher nuclear charge and slightly smaller atomic radius of Mg than Na.
After the loss of first electron, Na+ formed has the electronic configuration of neon (2, 8). The higher stability of the completely filled noble gas configuration leads to very high second ionisation enthalpy for sodium.
On the other hand, Ma+ formed after losing first electron still has one more electron in its outermost (3s) orbital. As a result, the second ionisation enthalpy of magnesium is much smaller than that of sodium.
Question 3. Give reasons:
(i) IE1 of sodium is lower than that of magnesium whereas IE2 of sodium is higher than that of magnesium.
(ii) Noble gases have positive value of electron gain enthalpy.
Answer: (i) The effective nuclear charge of magnesium is higher than that of sodium. For these reasons, the energy required to remove an electron from magnesium is more than the energy required in sodium. Hence, the first ionization enthalpy of sodium is lower than that of magnesium.
However, the second ionization enthalpy of sodium is higher than that of magnesium.
This is because after losing an electron, sodium attains the stable noble gas configuration. On the other hand, magnesium, after losing on electron still has one electron.
(ii)Because of stable configuration.
Here we presented you with everything about CBSE NCERT Solutions For Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties. Still, if you find any sort of doubts makes sure to ask us since are always here to help you out.
FAQ: NCERT Solutions For Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
Can I download NCERT Solutions For Class 11 Chemistry Chapter 3 for free?
Yes, you can download NCERT Solutions For Class 11 Chemistry Chapter 3 for free.
What is the name of Chapter 3 in chemistry class 11?
Classification of Elements and Periodicity in Properties is the third chapter in the NCERT Solutions For Class 11 Chemistry Chapter 3 syllabus.
What are the topics included in Periodic Trends In Properties Of Elements of NCERT Solutions For Class 11 Chemistry Chapter 3?
Trends in Physical Properties
Periodic Trends In Chemical Properties
Periodic Trends And Chemical Reactivity
What is periodic properties NCERT Solutions For Class 11 Chemistry Chapter 3?
The basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number.
These properties reappear at regular intervals or follow a particular trend at regular intervals. This phenomenon is known as the periodicity of elements.
How many elements are present now?
At present, 118 elements are known to us.